Abstract
Introduction: Chimeric antigen receptor (CAR) T-cell therapy has shown promising efficacy in patients with relapsed/refractory (r/r) large B-cell lymphoma (LBCL) although responses and survival probability are highly variable across patients. High tumor burden before CAR T-cell administration has been associated with an inferior survival. Recently, radiomic features extracted from PET/CT scans have shown to be predictive of outcomes in patients with LBCL treated with conventional chemotherapy, but data in the setting of CAR T-cell therapy remains scarce.
Methods: We included consecutive patients with r/r LBCL treated at a single institution with commercially approved CAR T-cell products and with an available PET/CT scan performed within the week starting lymphodepleting chemotherapy. Lesions were delineated using a semi-automated preselection of 18F-FDG avid lesions. Quantifiable radiomic features, including first order, shape and texture matrices from the Standard Uptake Values (SUV) of PET images were extracted from the largest lesion. Conventional PET features including total metabolic tumor volume (TMTV) and maximum SUV (SUVmax) were acquired from the whole metabolic disease. Data was split into training and test sets (80-20%) which were balanced for treatment response, type of construct and IPI score. Feature selection was performed using the least absolute shrinkage and selection operator (LASSO)-regression. A logistic regression model was trained and test for predicting clinical benefit defined as progression-free survival (PFS) >3.2 months (median). The performance of clinical characteristics, conventional PET features and the radiomics score to predict clinical benefit were compared using the area under the curve (AUC) from the receiver operating characteristic curves. Univariable and multivariable survival Cox models were evaluated for continuous PFS and overall survival (OS) including the selected variables from the LASSO-Cox models.
Results: Ninety-three patients were included in the study. Median age was 59 years (IQR 50-68) and 68% were male. Median number of prior lines was 2 (IQR 2-3). Seventy-three percent of patients had an advance stage (III-IV) and 30% had a previous autologous stem cell transplant. Regarding the construct, 33% received a CAR with a CD28 costimulatory domain and 67% with a 4-1BB.
Best response after CAR T-cell therapy was complete remission in 37 (40%) patients, partial remission in 33 (35%) and progressive disease in 23 (25%). The median PFS and OS for the whole group were 3.27 (IQR 2.27-10.43) and 8.87 (IQR 4.60-15.40) months, respectively. In terms of toxicity, twenty-nine (31%) and 25 (27%) patients presented grade ≥2 cytokine release syndrome (CRS) and grade ≥2 neurotoxicity, respectively.
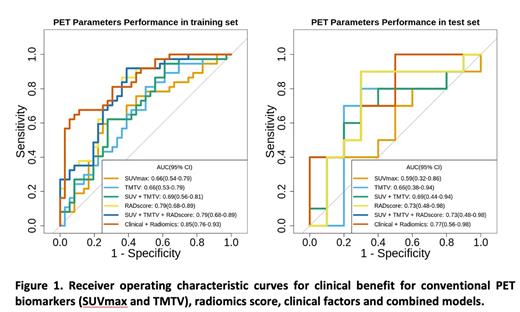
Four features related to SUV, size and heterogeneity were selected by the LASSO-regression method and constitute the radiomic signature. This signature was predictive of clinical benefit in the training and test sets (AUC of 0.79 and 0.73, respectively). Also, the radiomic signature outperformed conventional PET established markers including SUVmax (AUC=0.59) and TMTV (AUC= 0.66), individually or in combination (AUC= 0.69). After combining the radiomic signature with clinical variables selected by LASSO, the AUC improved to 0.85 and 0.77 in the training and test sets, respectively (Figure 1). In the multivariate analysis including clinical, classical PET and radiomic variables the only 2 factors associated with higher PFS were CAR T-cell product (axicabtegene ciloleucel) (HR 0.30, 95% CI [0.13-0.7], p= 0.005) and a favorable radiomic signature (HR 0.09, 95% CI [0.02-0.7], p <0.001). Lower ECOG performance status score (HR 0.32, 95% CI [0.13-0.75], p=0.009) and a favorable radiomic signature (HR 0.03, 95% CI [0.01-0.14], p<0.001) were significantly associated with prolonged OS.
Finally, the radiomic signature or classical PET parameters were not associated with higher incidences of CRS or neurotoxicity.
Conclusions: Prediction models using 18F-FDG PET/CT radiomic features at baseline aid in identify LBCL patients benefiting from CD19-targeted CAR T-cell therapy. The positive predictive value increases when clinical parameters are added to the radiomic features.
Disclosures
Barba:Gilead: Honoraria; Amgen: Honoraria; Novartis: Honoraria; BMS: Honoraria; Pfizer: Honoraria. Carpio:Regeneron: Honoraria; AstraZeneca: Honoraria; Kite/Gilead: Honoraria; Novartis: Honoraria. Abrisqueta:AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Sandoz: Honoraria; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees. Bosch:Novartis: Consultancy, Honoraria; Celgene/BMS: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Karyospharm: Honoraria; Roche: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria; Mundipharma: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Lilly: Consultancy, Honoraria; Beigene: Consultancy, Honoraria. Iacoboni:NOVARTIS, KITE/GILEAD, BMS/CELGENE, ASTRAZENECA, ROCHE, ABBVIE, JANSSEN, MILTENYI: Honoraria; NOVARTIS, KITE/GILEAD, BMS/CELGENE: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal